This image and description give details on how the influenza virus has evolved in clusters. Seasonal vaccinations increase the distance between clusters by selecting against certain strains.
Smith, D.J., A.S. Lapedes, J.C. de Jong, T.M. Bestbroer, G.F. Rimmelswaan, A.D.M.E. Osterhaus et.al. Mapping the Antigenic and Genetic Evolution of Influenza Virus. Science Magazine. 305(5682): 371-376, 2004.
Hannah Millimet
The Evolution of Illness
Wednesday, March 6, 2013
Friday, February 22, 2013
Cholera
V. cholerae, bacteria responsible for cholera.
Photo taken from www.npr.org/blogs/health
Vibrio cholerae, the bacteria responsible for the disease cholera, claims over one hundred thousand lives every year and can spread explosively among populations. The virus is highly transmittable and must be ingested to spread infection. Many people act merely as carriers of the disease meaning they house the bacteria in their bodies but display no symptoms of infection, making the disease very difficult to eradicate. For those individuals unlucky enough to succumb to their bacterial guests, death comes as a result of dehydration caused by excessive watery diarrhea. The world has experienced 7 separate cholera pandemics since the 19th century.
http://nationalpostcomment.files.wordpress.com/2010/11/cholera1125.jpg
Due to its virulent nature and significant impact on human health and history, cholera has been studied and observed by the scientific community for quite some time. Scientists have developed used cholera to develop vaccines as well as learn more about the spread of diseases and the rapidity of bacterial evolution among infectious diseases. Much like Streptococcus pneumoniae, the bacteria responsible for diseases like pneumonia, V. cholerae evolves quickly to produce more effective and dangerous strains to infect human hosts. While the tendency of cholera causing bacteria to evolve rapidly may appear to have only negative implications, it actually helps epidemiologists track and control potential outbreaks. Scientists can draw on DNA databases filled with the genetic code for all current and past strains of V. cholerae to use as comparison with new outbreaks of the disease. This allows governments to determine the cause and track the spread of the disease in order to contain and treat it. Recently this method of using genetic information to compare strains of V. cholerae helped the Haitian government track the cause of the recent 2010 outbreak of cholera to UN workers from Nepal. (http://www.bbc.co.uk/news/world-latin-america-21542842)
Sources:
Is Pertussis Evolving to Fight Vaccines?
Pertussis, more commonly known as whooping
cough, has unfortunately shown a strong resurgence within the past few years
not only in the United States, but in countries across the globe as well. In fact, there were a reported 27, 550 cases
of pertussis in the United States in 2010, marking the year as that with the
most numerous cases of the infection since 1959. This new-found prominence in the disease has in
turn resulted in a variety of public service announcements, on the radio and
television, warning the general public of the symptoms and the importance of
vaccination, especially for those with infants, as they tend to be the group most
susceptible to the infection. Without a
doubt, the most resonating part of the segments are the sound bites of infants
first going into coughing fits before beginning to gasp for air, the
characteristic whooping sound, placed intermittently throughout the duration of
the message. If the information in the
public service announcement does not catch your attention, the pained sounds of
sick infants definitely will.
Pertussis is a highly contagious respiratory disease caused by an
infection by the bacteria Bordetella
pertussis. The bacteria attack the
upper respiratory system, releasing toxins that cause inflammation. This in turn causes those infected to descend
into bursts of rapid coughs which are then followed by a period of attempting
to draw in as much air as possible in order to recover from the depletion in
oxygen levels in the body due to the long coughing spells. The disease often leads to the development of
pneumonia, and in the worst cases the depletion in oxygen levels can lead to
brain damage and even death. For this reason,
physicians highly recommend vaccination to those in direct contact with infants
as it has shown to significantly decrease the chance of spreading the disease.
Despite the effectiveness of vaccination, public health studies have
shown that pertussis has again begun to reemerge, but unexpectedly in countries
with highly vaccinated populations.
Though factors such as decreases in vaccine coverage and vaccine quality
could play a role in this resurgence of the disease, researchers in the
Netherlands hypothesize that the bacteria has evolved to resist the effects of
vaccines.
To test this theory, the researchers collected bacteria strains from
1949 to 1996, grouping them into periods of 5 to 8 years, and determined the frequency
of different DNA fingerprint types within each of said periods. The results showed a distinct difference
between in the fingerprints types found before and after usage of the vaccine
became widespread. Moreover, the results
showed that genotypic diversity drastically decreased soon after implementation
of the vaccine, suggesting that only those bacteria with the correct genetic coding
were able to resist being wiped out.
However, over the years the genotypic diversity has increased, implying that
those surviving strains have continued to adapt and mutate so as to remain
unaffected by vaccines.
In addition, the researcher also investigated the effects of the polymorphism
observed in pertussis toxin and pertactin, two important virulence factors
necessary for the bacteria to be able to bind to the host’s cells. Results showed the polymorphisms were non-conservative
for the most part, which would imply that Darwinian selection plays an important
role in this adaption found in the bacterial DNA. Specifically, results showed that tandem
repeats in the coding existed near the RGD amino acid motif, making the area
quite susceptible to mutation as a result of slipped-strand mispairing during
DNA replication. Said mutations affect
areas of the bacteria related to binding with T-cells, causing those bacteria
with sequences most distinct from those found in the pre-vaccination era
selected to be selected. Because of the
mutations, the receptor binding area has changed such that the host’s T-cells
can no longer bind to the bacteria, meaning that the bacteria no longer has to
fear being eliminated from the host.
Though the usage of vaccines to help fight the spread of pertussis has
without a doubt been quite beneficial at reducing the number of pertussis-related
deaths over the decades, it would seem that yet again the bacteria are adapting
and evolving to resist the effects of said vaccines. Those bacteria able to survive the initial wave
of vaccines have evolved and given way to new mutants that are beginning to
show resistance against T-cells. Though
this is most likely not the only reason why pertussis has shown an increase in
activity within the past few years, it does open up more doors towards the
continued effort to eventually bring immunity towards the disease hopefully sometime
in the future.
Danielle Spencer
Word Count: 760
References:
1. http://www.cdc.gov/pertussis/
2. Mooi, Frits R., Inge H. M. van Loo, Audrey J.
King. Adaptation of Bordetella pertussis
to Vaccination: A Cause for Its Reemergence? Emerging Infectious Diseases.
7(3, Supplement): 526-528, 2001.
Image from: http://medblog.medlink-uk.net/gangnamlad/files/2013/02/whooping-cough.jpg
A Race to Evolve - The Evolution of HIV in Response to Pharmaceuticals by Jesse Passman
One
of the most publicized diseases of this day and age is the Human
Immunodeficiency Virus, or HIV. Since
its emergence a few decades ago, the rise of its infection rates and total
occurrence have been a concern, especially in developing nations. HIV today infects 50,000 new individuals in
the U.S. a year and the CDC estimates that 1,150,000 people area already
infected in the U.S. While many diseases
that plague humanity have effective vaccines available, HIV still does
not. Treatments have been created for
HIV infection; however they rarely work for long due to quick evolution of the
virus. This is concerning to all parties
involved as without an effective cure or disease control program, the disease
is destined to continue its spread.
Word Count: 539
Sources:
Clavel, Fracois, and Allan J. Hance. "HIV drug resistance." New England Journal of Medicine 350.10 (2004): 1023-1035.
"HIV/AIDS Statistics and Surveillance." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 19 December 2012. Web. 20 February 2013.
Image source: http://upload.wikimedia.org/wikipedia/commons/1/1a/HIV-budding-Color.jpg
HIV particles (green) budding from a cell.
HIV,
which is spread through blood, semen, and other bodily fluids, is a prime
example disease evolution in the modern age.
Its rapid and progressive change in the face of attempted cures has
baffled scientists for years. For
instance, after the first antiretroviral drug active against HIV, zidovudine,
came out, resistant HIV strains were found in new patients within six years. But one may wonder why HIV is so much better
at evolving in response to drugs than other bacteria and viruses. The answer is two-fold. The first aspect comes from just how virulent
HIV is. Its production of new virus and overall
virus turnover is extraordinary.
According to Clavel and Hance, the lymphoid tissue of most untreated
patients has between 107 and 108 infected cells, each of
which has a half-life of one to two days.
To maintain this level, HIV must infect many new cells
continuously.
The second aspect is the rate of mutation between replications of the virus. When HIV infects a human cell, it hijacks its machinery to create more HIV. The reverse transcription process it uses, however, is very error prone. For each copy of the virus that is created, at least one error occurs – a mutation. While this may not seem significant, when it is extrapolated over the huge population of HIV found in someone’s body, it leads to a diverse set of HIV particles with a diverse set of traits. Some of them are weaker than your average virus; some are stronger and more effective.
The second aspect is the rate of mutation between replications of the virus. When HIV infects a human cell, it hijacks its machinery to create more HIV. The reverse transcription process it uses, however, is very error prone. For each copy of the virus that is created, at least one error occurs – a mutation. While this may not seem significant, when it is extrapolated over the huge population of HIV found in someone’s body, it leads to a diverse set of HIV particles with a diverse set of traits. Some of them are weaker than your average virus; some are stronger and more effective.
Word Count: 539
Sources:
Clavel, Fracois, and Allan J. Hance. "HIV drug resistance." New England Journal of Medicine 350.10 (2004): 1023-1035.
"HIV/AIDS Statistics and Surveillance." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 19 December 2012. Web. 20 February 2013.
Image source: http://upload.wikimedia.org/wikipedia/commons/1/1a/HIV-budding-Color.jpg
The Evolution of Influenza
In depth
studies of the evolution of the influenza virus have shown interesting trends.
The strains tend to group in clusters rather than form a continuous antigenic
lineage. This is based on the seasonal immunizations, which select against
certain strains. There is a selective advantage for clusters farthest from
those in the vaccination.
 The quick
evolution of influenza poses a seemingly endless battle to the immune system.
Influenza epidemics are estimated to cause 500,000 deaths worldwide per year. Certain
strains of influenza have made their mark on human history by proving
particularly fatal. These include the Spanish flu of 1918 that killed an
estimated 50 million people worldwide, the Asian flu of 1957 that killed 2
million people, the Hong Kong flu in 1968 that killed 1 million people, and the
swine flu of 2009, which killed an estimated 295,000 people. The global impact
of this virus proves the importance of predicting its evolution to the best of
our abilities in order to develop effective vaccinations. However, much of the
evolution of influenza is due to random and unpredictable reassortments and
genetic drifts, thereby making this task difficult.
The quick
evolution of influenza poses a seemingly endless battle to the immune system.
Influenza epidemics are estimated to cause 500,000 deaths worldwide per year. Certain
strains of influenza have made their mark on human history by proving
particularly fatal. These include the Spanish flu of 1918 that killed an
estimated 50 million people worldwide, the Asian flu of 1957 that killed 2
million people, the Hong Kong flu in 1968 that killed 1 million people, and the
swine flu of 2009, which killed an estimated 295,000 people. The global impact
of this virus proves the importance of predicting its evolution to the best of
our abilities in order to develop effective vaccinations. However, much of the
evolution of influenza is due to random and unpredictable reassortments and
genetic drifts, thereby making this task difficult.
Hannah Millimet
Word count: 356
References:
1. http://www.historyofinfluenza.com/
2. Smith, D.J., A.S. Lapedes, J.C. de Jong, T.M. Bestbroer,
G.F. Rimmelswaan, A.D.M.E. Osterhaus et.al. Mapping
the Antigenic and Genetic Evolution of Influenza Virus. Science Magazine.
305(5682): 371-376, 2004.
Images from:
1. http://medimoon.com/2012/08/fda-approves-vaccines-for-the-2012-2013-influenza-season/
2. http://en.wikipedia.org/wiki/1918_flu_pandemic
Wednesday, February 20, 2013
Why the hereditary disease hemochromatosis persists
Survival of the Sickest: the Surprising
Connections between Disease and Longevity by Dr. Sharon Moalem and
Jonathan Price explores the possible evolutionary pressures which have
preserved diseases such as hemochromatosis, Type 1 diabetes, high cholesterol
levels, and favism. The book gave interesting insights as to why diseases which
are deadly today may have been potentiated. While the authors explored several
different diseases, this review will focus on the explanations presented for
the persistence of hemochromatosis.
Hemochromatosis:
Hemochromatosis,
also called iron overload, is a hereditary condition which occurs when the body
absorbs too much iron. The result is the accumulation of iron in organs such as
the heart, liver, and pancreas, which in turn can lead to complications,
including diabetes, liver failure, and heart failure. The symptoms of
hemochromatosis can be alleviated through bloodletting, thereby reducing the
amount of iron present, or through chelating agents which bind to iron and then
may be excreted. If left untreated, hemochromatosis can be deadly.
Dr.
Moalem presents the hypothesis that hemochromatosis, while deadly today,
originally protected people from the bubonic plague. Iron is targeted by
bacteria, viruses, parasites, and cancer. Disease causing agents require it to
persist, and they find it in the human tissue they infect. To combat this, iron
is “locked up” when humans become sick in an effort to prevent the disease-causing
organism from being able to survive. While people suffering from
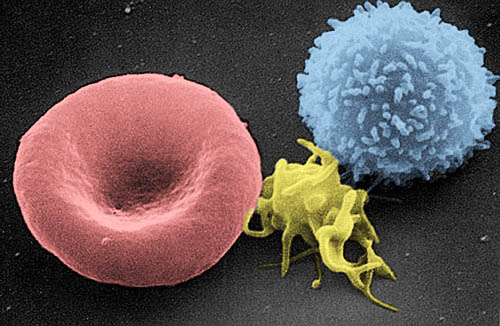
hemochromatosis have an excess of iron in many tissues, there is less iron than
normal present in macrophages, a type of white blood cell. Because the bubonic
plague utilized macrophages to spread through the body using the lymphatic
system, people who had less iron present in macrophages were less likely to
have the plague survive and multiply within the macrophages before it reached
the lymph nodes. These people where therefore more likely to survive and pass
on the gene for hemochromatosis.
The
idea that iron-deficient macrophages are better at combating bacteria has been
tested both in vivo and in vitro. In cell culture,
iron-deficient macrophages are much more capable of successfully overcoming
bacteria. Somali nomads who traditionally have had anemia became more
susceptible to infection when given iron supplements.
During
the period of the Black Plague, one of the most famous outbreaks of the bubonic
plague, young, healthy men were more likely to die than any other group. At the
time, younger men would have been the least likely to have iron-deficiencies,
and thus the most vulnerable to the plague. While this is not conclusive
evidence as to why hemochromatosis was sustained within the population, it is
an interesting hypothesis which is at least partially supported by studies
today.
Claire
Klimko
Word
count: 440 words
Reference:
Moalem,
Sharon and Jonathan Prince. Survival of
the Sickest: the Surprising Connections between Disease and Longevity. New
York: HarperCollins, 2007.
Image from http://en.wikipedia.org/wiki/File:Red_White_Blood_cells.jpg
Sunday, January 13, 2013
Welcome!
Welcome to the Process of Evolution! During this semester, we hope to explore how evolution affects and shapes our daily lives through our class and this blog.
Subscribe to:
Posts (Atom)